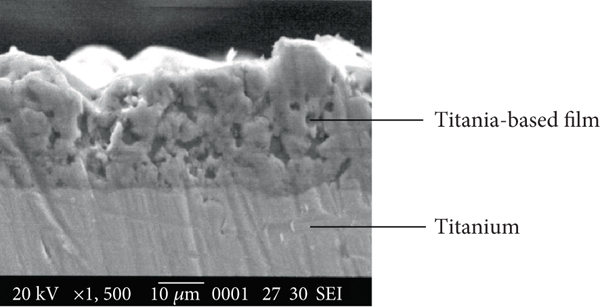
The use of titanium dioxide (TiO2) in factory settings is widespread, with this versatile compound playing a crucial role in various industrial processes. TiO2 is a naturally occurring mineral that is widely used as a white pigment in paints, coatings, plastics, paper, and other products. Its ability to effectively scatter light makes it an ideal choice for creating bright, durable, and long-lasting finishes.
- There are several factors that can influence the cost of titanium dioxide from suppliers. One of the main factors is the availability of raw materials, specifically titanium ore. The cost of extracting and processing titanium ore can fluctuate, which can in turn affect the cost of titanium dioxide. Suppliers must also take into consideration the cost of energy and other resources needed for the production of titanium dioxide, such as labor and equipment.
However, humans are not exposed to E171 in drinking water at any significant quantity over a long duration, so this potential effect is irrelevant to the human experience. It’s important to understand that a potential hazard is not the same thing as an actual risk.
 cheap barium sulphate superfine factory. This is typically achieved through a combination of grinding and classification techniques. The resulting barium sulfate powder is then dried and packaged for distribution.
cheap barium sulphate superfine factory. This is typically achieved through a combination of grinding and classification techniques. The resulting barium sulfate powder is then dried and packaged for distribution.Exposure routes are the pathways that allow ingredients to enter our bodies. Primary exposure routes include:
4. Cost-Effectiveness Purchasing titanium dioxide in wholesale quantities can lead to significant cost savings for tire manufacturers. By acquiring TiO2 in bulk, manufacturers can reduce production costs per unit, thereby improving their profit margins. Moreover, the durability and performance enhancements associated with TiO2 help reduce the frequency of tire replacements, further amplifying cost efficiency.
 titanium white oem supplier. We understand that each of our customers has unique needs, and we strive to provide personalized support to ensure that you get the best possible experience. Our experienced team of professionals is always available to answer your questions, provide technical support, and help you select the right product for your specific application.
titanium white oem supplier. We understand that each of our customers has unique needs, and we strive to provide personalized support to ensure that you get the best possible experience. Our experienced team of professionals is always available to answer your questions, provide technical support, and help you select the right product for your specific application.≥ 5 % of standard sample
Technical Specifications:(Standard:Q/SNBJ1-2012)
Titanium dioxide (TiO2) is considered as an inert and safe material and has been used in many applications for decades. However, with the development of nanotechnologies TiO2 nanoparticles, with numerous novel and useful properties, are increasingly manufactured and used. Therefore increased human and environmental exposure can be expected, which has put TiO2 nanoparticles under toxicological scrutiny. Mechanistic toxicological studies show that TiO2 nanoparticles predominantly cause adverse effects via induction of oxidative stress resulting in cell damage, genotoxicity, inflammation, immune response etc. The extent and type of damage strongly depends on physical and chemical characteristics of TiO2 nanoparticles, which govern their bioavailability and reactivity. Based on the experimental evidence from animal inhalation studies TiO2 nanoparticles are classified as “possible carcinogenic to humans” by the International Agency for Research on Cancer and as occupational carcinogen by the National Institute for Occupational Safety and Health. The studies on dermal exposure to TiO2 nanoparticles, which is in humans substantial through the use of sunscreens, generally indicate negligible transdermal penetration; however data are needed on long-term exposure and potential adverse effects of photo-oxidation products. Although TiO2 is permitted as an additive (E171) in food and pharmaceutical products we do not have reliable data on its absorption, distribution, excretion and toxicity on oral exposure. TiO2 may also enter environment, and while it exerts low acute toxicity to aquatic organisms, upon long-term exposure it induces a range of sub-lethal effects.
Do you want to find out more about the chemicals market in China? Join our professional online platform today and get insights in Reports, Newsletter, and Market Data at one place. For more trade information on TiO2 visit our experts in trade analysis to get your answers today.
 This competition forced many factories to adapt or risk closure This competition forced many factories to adapt or risk closure
This competition forced many factories to adapt or risk closure This competition forced many factories to adapt or risk closure pigment lithopone factories. Some chose to specialize in niche markets where lithopone's unique characteristics were highly valued, while others focused on improving their production processes to reduce costs.
pigment lithopone factories. Some chose to specialize in niche markets where lithopone's unique characteristics were highly valued, while others focused on improving their production processes to reduce costs.Titanium dioxide nanoparticles may accumulate and cause DNA damage
105°C volatile matter, %
Quality Titanium Dioxide Suppliers:
In a study published in the journal Toxicology, researchers examined the effects of exposing human colon cancer cell line (HTC116) titanium dioxide food additives in vitro. “In the absence of cytotoxicity, E171 was accumulated in the cells after 24 hours of exposure, increasing granularity and reactive oxygen species, inducing alterations in the molecular pattern of nucleic acids and lipids, and causing nuclei enlargement, DNA damage and tubulin depolymerization,” the scientists wrote. Researchers removed the additive from the culture, then examined the results 48 hours later. They found, “The removal of E171 was unable to revert the alterations found after 24 h of exposure in colon cells. In conclusion, exposure to E171 causes alterations that cannot be reverted after 48 h if E171 is removed from colon cells.”
Recently, Yanagisawa et al. reported that the transdermal exposure (mimicking skin-barrier dysfunction or defect) of NC/Nga mice to TiO2 NPs (15, 50, or 100 nm), in combination with allergen, aggravated atopic dermatitis-like lesions through a T-helper type 2 (Th2) dominant immune response. The study also indicated that TiO2 NPs can play a role in the initiation and/or progression of skin diseases, since histamine was released, even in the absence of allergen.
Recent policy changes in regard to titanium dioxide
Lithopone is a white pigment composed of a mixture of barium sulfate (BaSO4) and zinc sulfide (ZnS). It is commonly used in the production of paints, plastics, rubber, and various other industrial applications. As such, manufacturers and distributors often provide Material Safety Data Sheets (MSDS) to ensure the safe handling and use of the product.
Titanium dioxide in food