anatase titanium dioxide in coatings factories
...
2025-08-15 08:17
2076
Another classification lies in the scale of operation. Large-scale calcium carbonate factories, often equipped with advanced machinery and automation, cater to the demands of the global market. In contrast, small-scale or local factories, while having a lower production capacity, might serve regional needs or specialize in niche products.
...
2025-08-15 07:53
1808
...
2025-08-15 07:37
258
On the other hand, the U.S. Food and Drug Administration (FDA) in their Final Administrative Order on Sunscreen Drug Products posted in September 2021 still accepts titanium dioxide up to 25% in the list of Generally Recognized As Safe and Effective (GRASE) in the main document, without further clarification on what kind or size of particles [9]. However, on page 24 (Sunscreen containing nanomaterials) FDA clearly “distinguish nanomaterials from other forms of these ingredients'' (zinc oxide and titanium dioxide) and ask for comments on “any particular nanomaterials that you believe should not be permitted for use in OTC sunscreen products”. To the best of our knowledge, this Agency did not ban the use of nanoparticulate titanium dioxide in any form, even though it is mentioned on page 34 that the anatase form is the more photoactive one, due to the lack of evidence with real sunscreens OTC (over the counter) in vivo. Moreover, other regulations in Latin America (MERCOSUR agreement, 2006) do not state clearly their position on the use of nanoparticulate TiO2NPs [10].
...
2025-08-15 07:36
811
In conclusion, the rutile market factory industry plays a vital role in meeting the growing demand for this versatile material. With its excellent physical and chemical properties, rutile is,、。,。,,。
...
2025-08-15 07:23
1653
...
2025-08-15 07:08
863
China has emerged as a significant player in the global talc and titanium dioxide market, contributing to the production, consumption, and export of these essential minerals. Talc, also known as talcum powder, is a naturally occurring mineral that is widely used in various industries, including papermaking, plastics, rubber, cosmetics, and pharmaceuticals. Titanium dioxide, on the other hand, is a white pigment that is primarily used in paints, coatings, plastics, and paper. Both minerals have unique properties that make them indispensable in numerous applications.
...
2025-08-15 06:40
1848
In the dynamic world of cutting-edge technology, certain components play an indispensable role. Among these essential elements are the suppliers of R960 TIO2%, a specialized compound with a myriad of applications across various industries. These suppliers form the backbone of innovation, ensuring that the demand for this crucial component is met with precision and reliability.
...
2025-08-15 06:31
2689
Particle size = 0.3-0.5 micrometers
...
2025-08-15 06:19
1665
Another classification lies in the scale of operation. Large-scale calcium carbonate factories, often equipped with advanced machinery and automation, cater to the demands of the global market. In contrast, small-scale or local factories, while having a lower production capacity, might serve regional needs or specialize in niche products.
...
2025-08-15 07:37
258
On the other hand, the U.S. Food and Drug Administration (FDA) in their Final Administrative Order on Sunscreen Drug Products posted in September 2021 still accepts titanium dioxide up to 25% in the list of Generally Recognized As Safe and Effective (GRASE) in the main document, without further clarification on what kind or size of particles [9]. However, on page 24 (Sunscreen containing nanomaterials) FDA clearly “distinguish nanomaterials from other forms of these ingredients'' (zinc oxide and titanium dioxide) and ask for comments on “any particular nanomaterials that you believe should not be permitted for use in OTC sunscreen products”. To the best of our knowledge, this Agency did not ban the use of nanoparticulate titanium dioxide in any form, even though it is mentioned on page 34 that the anatase form is the more photoactive one, due to the lack of evidence with real sunscreens OTC (over the counter) in vivo. Moreover, other regulations in Latin America (MERCOSUR agreement, 2006) do not state clearly their position on the use of nanoparticulate TiO2NPs [10].
...
2025-08-15 07:36
811
In conclusion, the rutile market factory industry plays a vital role in meeting the growing demand for this versatile material. With its excellent physical and chemical properties, rutile is,、。,。,,。
...
2025-08-15 07:23
1653
On the other hand, the U.S. Food and Drug Administration (FDA) in their Final Administrative Order on Sunscreen Drug Products posted in September 2021 still accepts titanium dioxide up to 25% in the list of Generally Recognized As Safe and Effective (GRASE) in the main document, without further clarification on what kind or size of particles [9]. However, on page 24 (Sunscreen containing nanomaterials) FDA clearly “distinguish nanomaterials from other forms of these ingredients'' (zinc oxide and titanium dioxide) and ask for comments on “any particular nanomaterials that you believe should not be permitted for use in OTC sunscreen products”. To the best of our knowledge, this Agency did not ban the use of nanoparticulate titanium dioxide in any form, even though it is mentioned on page 34 that the anatase form is the more photoactive one, due to the lack of evidence with real sunscreens OTC (over the counter) in vivo. Moreover, other regulations in Latin America (MERCOSUR agreement, 2006) do not state clearly their position on the use of nanoparticulate TiO2NPs [10].
In conclusion, the rutile market factory industry plays a vital role in meeting the growing demand for this versatile material. With its excellent physical and chemical properties, rutile is,、。,。,,。
China has emerged as a significant player in the global talc and titanium dioxide market, contributing to the production, consumption, and export of these essential minerals. Talc, also known as talcum powder, is a naturally occurring mineral that is widely used in various industries, including papermaking, plastics, rubber, cosmetics, and pharmaceuticals. Titanium dioxide, on the other hand, is a white pigment that is primarily used in paints, coatings, plastics, and paper. Both minerals have unique properties that make them indispensable in numerous applications.
In the dynamic world of cutting-edge technology, certain components play an indispensable role. Among these essential elements are the suppliers of R960 TIO2%, a specialized compound with a myriad of applications across various industries. These suppliers form the backbone of innovation, ensuring that the demand for this crucial component is met with precision and reliability.
Particle size = 0.3-0.5 micrometers
Titanium dioxide overnight news
By reducing processed foods in your diet, you can reduce the likelihood of not only eating titanium dioxide but eating other chemicals of concern, Faber said, noting that consumers can also call their elected representatives urging them to support increased food safety legislation and take action with organization alliances like Toxic Free Food FDA. America, once again, is falling behind the rest of the world when it comes to chemical safety.
 This makes the bearing suitable for use in precision machinery and other applications where noise and vibration can affect performance or reliability This makes the bearing suitable for use in precision machinery and other applications where noise and vibration can affect performance or reliability
This makes the bearing suitable for use in precision machinery and other applications where noise and vibration can affect performance or reliability This makes the bearing suitable for use in precision machinery and other applications where noise and vibration can affect performance or reliability Stainless steel and other corrosion-resistant alloys were developed to withstand harsh operating conditions, while improvements in manufacturing techniques allowed for the production of smaller, more precise bearings Stainless steel and other corrosion-resistant alloys were developed to withstand harsh operating conditions, while improvements in manufacturing techniques allowed for the production of smaller, more precise bearings
Stainless steel and other corrosion-resistant alloys were developed to withstand harsh operating conditions, while improvements in manufacturing techniques allowed for the production of smaller, more precise bearings Stainless steel and other corrosion-resistant alloys were developed to withstand harsh operating conditions, while improvements in manufacturing techniques allowed for the production of smaller, more precise bearings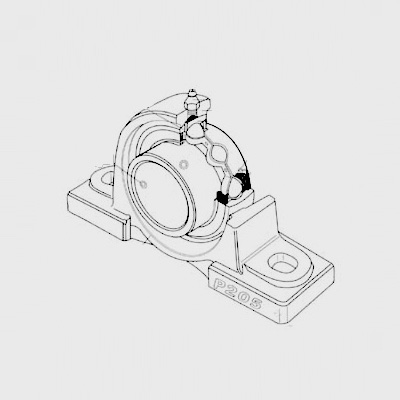
 This feature makes the bearing suitable for use in a variety of applications, including machinery, automotive, and aerospace industries This feature makes the bearing suitable for use in a variety of applications, including machinery, automotive, and aerospace industries
This feature makes the bearing suitable for use in a variety of applications, including machinery, automotive, and aerospace industries This feature makes the bearing suitable for use in a variety of applications, including machinery, automotive, and aerospace industries

 The width of the bearing can vary depending on the specific application, but it typically ranges from 7mm to 12mm The width of the bearing can vary depending on the specific application, but it typically ranges from 7mm to 12mm
The width of the bearing can vary depending on the specific application, but it typically ranges from 7mm to 12mm The width of the bearing can vary depending on the specific application, but it typically ranges from 7mm to 12mm