titanium dioxide dissolved in water manufacturers
Risk managers at the European Commission and in EU Member States have been informed of EFSA’s conclusions and will consider appropriate action to take to ensure consumers’ protection.
Testing samples were made mixing 100 uL of TiO2NPs suspensions (0.2 mg/mL and 0.02 mg/mL) and vitamins@P25TiO2NPs (0.2 mg/mL and 0.02 mg/mL) with 100 μL ATCC 29,213 methicillin-sensitive Staphylococcus aureus (MSSA) (107 in PBS, pH 7). Controls were made replacing nanoparticles with the same volume of PBS. The concentrations of nanoparticle suspensions were chosen based on the FDA approved maximal and the minimal amount usually found in sunscreens, which are 20% and 2% (this is equivalent to 0.2 mg/mL and 0.02 mg/mL for nanoparticles suspensions). The cream concentration, on the other hand, was an intermediate value of 10%.
There are two primary forms of titanium dioxide commercially available: anatase and rutile. The rutile form is typically used in sunscreens due to its superior ability to handle UV rays and stability in the presence of UV light. The anatase form is typically used in other types of products, such as paint. Another plus of the rutile form is that its UVA protection extends past 400 nanometers, which is the upper limit of UVA.
I claim, therefore, and desire to secure by these Letters Patent, the following:

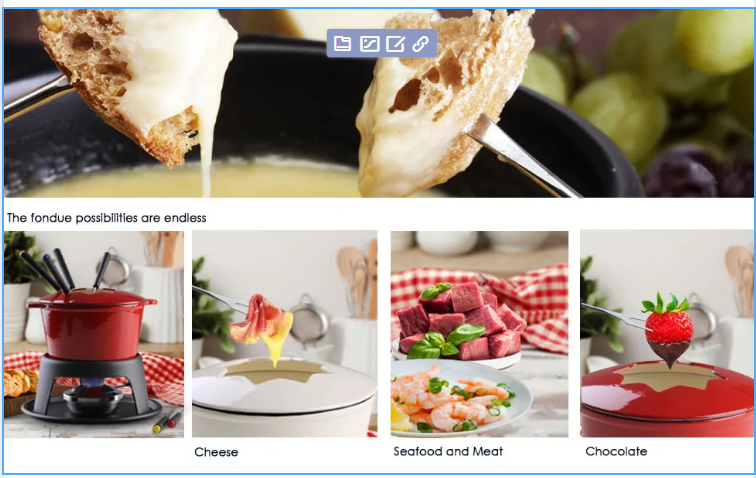 Its robust build allows it to go straight from stove to table, saving time and effort Its robust build allows it to go straight from stove to table, saving time and effort
Its robust build allows it to go straight from stove to table, saving time and effort Its robust build allows it to go straight from stove to table, saving time and effort
 These pots come in a variety of colors and designs, allowing you to choose one that complements your kitchen decor These pots come in a variety of colors and designs, allowing you to choose one that complements your kitchen decor
These pots come in a variety of colors and designs, allowing you to choose one that complements your kitchen decor These pots come in a variety of colors and designs, allowing you to choose one that complements your kitchen decor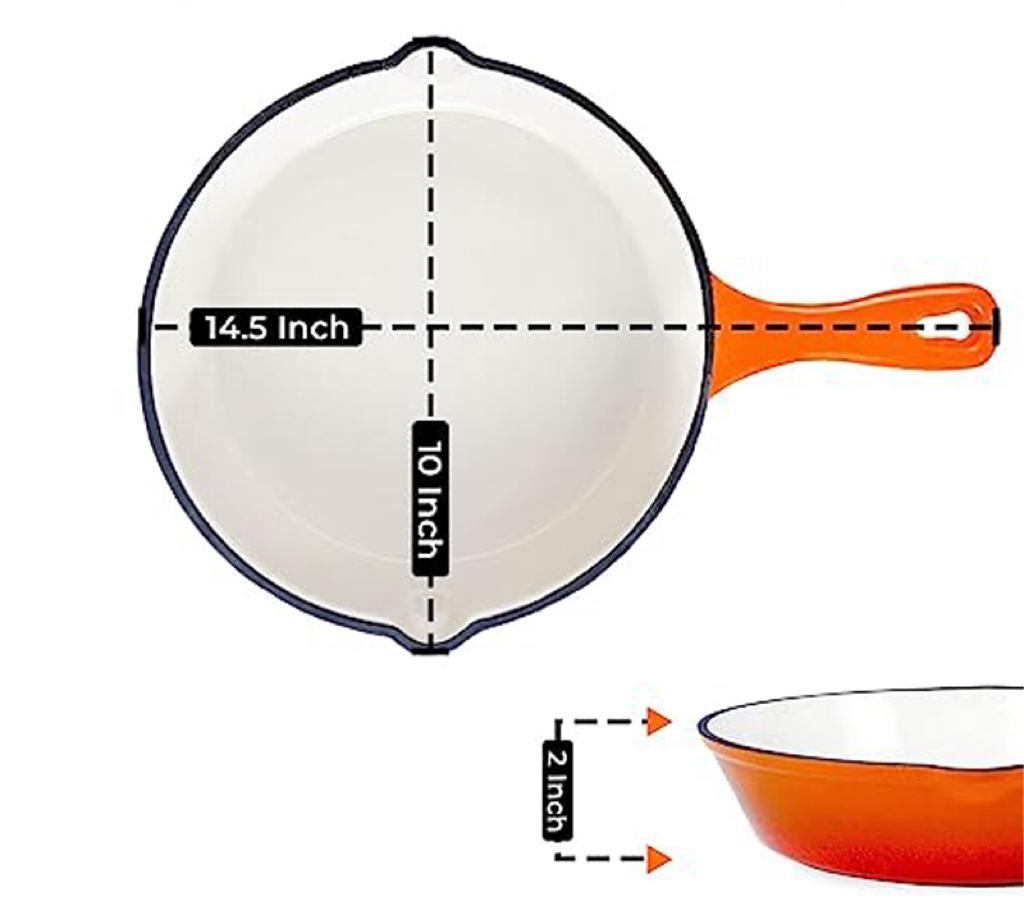 Allow it to cool completely in the oven to avoid warping Allow it to cool completely in the oven to avoid warping
Allow it to cool completely in the oven to avoid warping Allow it to cool completely in the oven to avoid warping
